

The aim of the LYO-CHECK project is to create two machines, one pilot machine and one fully automatic industrial machine, for the inspection of lyophilized pharmaceutical preparations intended for injection, based on the two elements of:
The two machines will be used for demonstration in operational environment with trials on potential customer’s products.


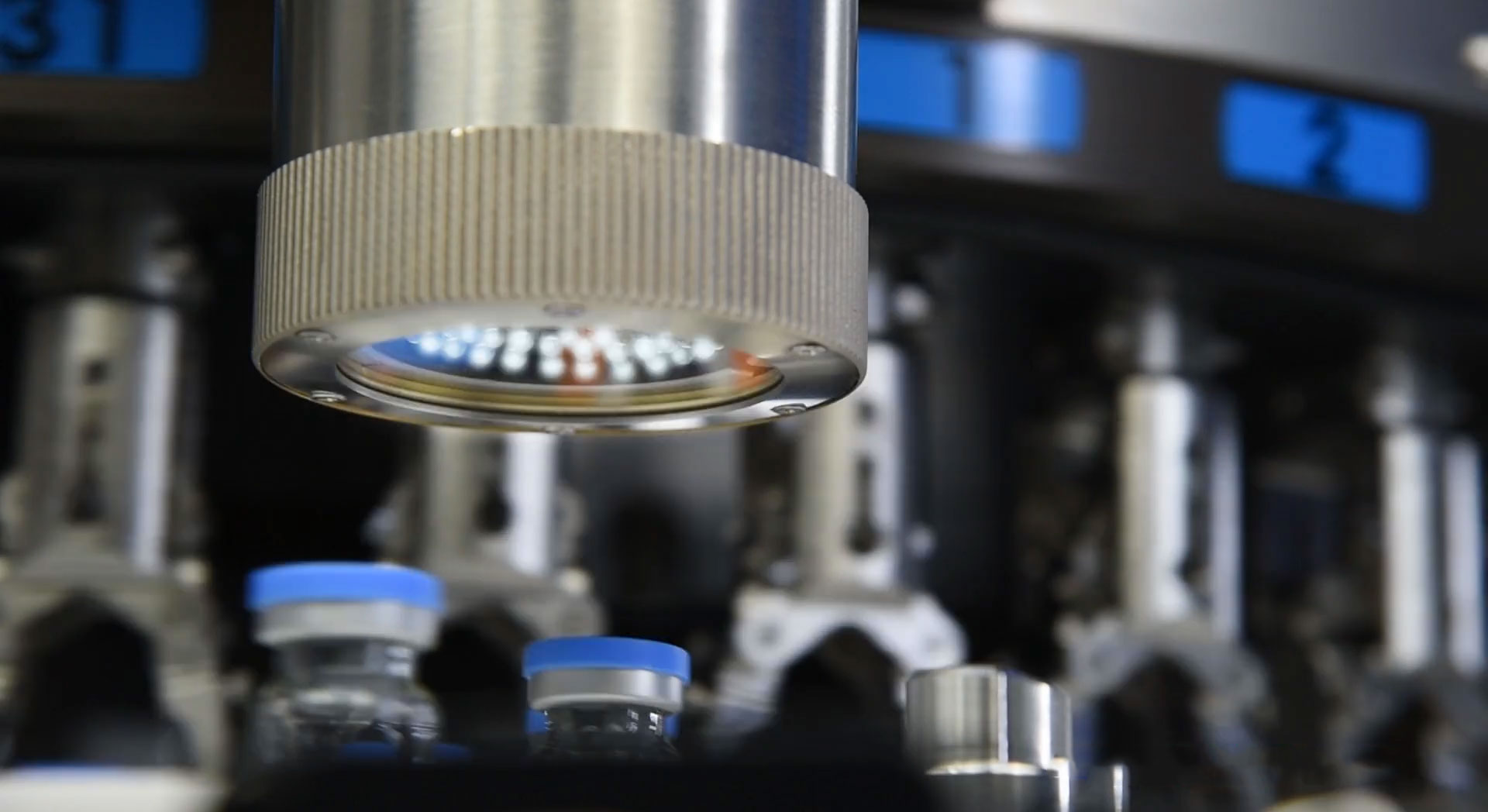
Pharmaceutical products intended for injection are becoming more and more commonly found in lyophilized form.
This trend appears to grow steadily, especially for anti-infectives, biotechnology derived products, and in-vitro diagnostics.
In fact, lyophilized product shows the following characteristics:
Nevertheless, the quality-control inspection of products in lyophilized form poses peculiar challenges, needing a dedicated system which is totally different from the one so far applied to inspect parenteral products in liquid form.
During the LYO-CHECK project, the new pilot and industrial machines will be used for demonstration in operational environment with trials on potential customer’s products.
The worldwide pharmaceutical market is driven by two main necessities:
Given that the lyophilized form is set to become the most widely used in the production of high added-value preparations for injection, the increase in product quality and production output can be achieved only by the combination of different factors and improvement strategies, which are being taken into account in the development of the LYO-CHECK machine.
The new automatic inspection process performed with the LYO-CHECK machine will impact the lyophilized products sector in terms of:

Antares Vision’s project for a disruptive innovation in the inspection technology for lyophilized products has been selected for funding within the European Union’s Horizon 2020 research and innovation programme thanks to:
General info:
Project Coordination:
Davide Sacchetti / Antares Vision
davide.sacchetti@antaresvision.com

